Projects
Neuroinflammation, Glutamatergic Signaling, and Nicotine Addiction
Neuroinflammation occurs with chronic drug use and has been implicated across a number of neuropsychiatric and neurodegenerative pathologies. We examine markers for neuroinflammation after nicotine addiction, during withdrawal, and during nicotine relapse. Further, we utilize a cre-recombinase driver transgenic rat line to chemogenetically activate or inhibit microglia, and examine their impact on nicotine neurobiology and behavior. Additionally, we evaluate causal relations between noninflammatory signals and rapid, transient, cue-induced synaptic plasticity in a nicotine addiction model. Due to the crucial roles glial cells have in these relations, analysis of microglial and astroglial cytokine production, morphology, and receptor expression is used to develop potential pharmocotherapeutic targets for nicotine addiction. This research is funded by a R01 from NIDA (2019-2024).
Neurobehavioral Impacts of Xylazine and Fentanyl Co-Use and Withdrawal
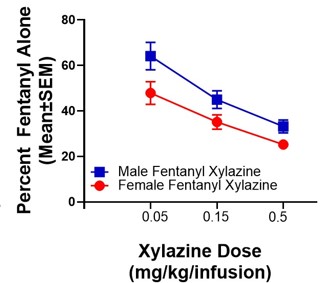
Prescription and illicit opioid use have risen to a public health crisis, with the landscape shifting to fentanyl use. Given that fentanyl is 100-fold more potent than morphine, its use is associated with a higher risk of fatal overdose. Naloxone (Narcan) reverses opioid overdose and precipitates withdrawal. However, recent reports indicate that xylazine, an anesthetic that is increasingly detected in accidental fentanyl overdose deaths, has become a highly sought-after adulterant that can prolong both the fentanyl “high” and the onset of fentanyl withdrawal, as well as precipitate resistance to naloxone in the reversal of overdose. Despite this, no systematic studies to date have evaluated the neurobehavioral mechanistic contributors to these effects. While opioid pharmacological mechanisms of action have been studied, it is not clear how the addition of xylazine to fentanyl inhibits the actions of naloxone, as either alone does not have this action. Excitingly, we have possibly discovered the mechanisms of how xylazine combined with fentanyl causes resistance to naloxone using our State-of-the-Art PamGene PamStation technology that measures hundreds of kinase activities in a single sample. We show preliminary data for the signaling pathways in the nucleus accumbens core (NAcore) that are hyper- or hypo-active, which gives us numerous potential targets for reversing naloxone resistance and xylazine’s effects on fentanyl self-administration (SA). We hypothesize that the xylazine-fentanyl combination activates non-canonical pathways that reduce consumption and prolong the onset of fentanyl withdrawal signs. Our objective here is to uncover these unknown mechanisms using our PamGene technology and determine how these impact neurocircuitry and behavior. Therefore, we will test these in our following Aims: 1) Determine how xylazine impacts the effects of fentanyl, 2) Determine viable therapeutic targets to resensitize fentanyl withdrawal following the escalation of xylazine-fentanyl SA, and 3) Determine neural circuits altering the NAcore and ventral tegmental area (VTA) kinomes following the escalation of xylazine-fentanyl SA and if these underlie naloxone resistance. We will map the active kinome of the NAcore and VTA following xylazine-fentanyl co-use and will test novel treatment targets that we find are changed via our extensive analysis. We will further test if these neural circuits contribute to co-use-induced alterations in targets that we have identified from our PamGene analysis, and thus we will determine if suppressing these circuits may enhance the actions of naloxone and restore neural targets altered by xylazine-fentanyl co-use (measured via kinome analysis). Together, these hypothesis-driven studies will markedly enhance our understanding of the neurobehavioral mechanisms underlying xylazine-fentanyl polysubstance use and withdrawal and will likely reveal novel treatment strategies for reversing the xylazine-fentanyl-induced naloxone inefficacy.
Steroidal Hormone Effects on Nicotine Addiction Vulnerability in Females
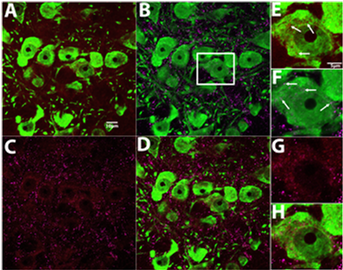
Women are typically more vulnerable to substance use disorders and clinical trials have shown that long-term smoking cessation is more difficult to achieve in women than men. Clinical research studies suggest that the menstrual cycle phase in women can affect cigarette craving and propensity to relapse to smoking following abstinence. Our lab explores hormonal influences on glutamatergic and neuroimmune signaling mechanisms in addiction. Our investigations of hormonal involvement in the pathways that underlie drug-seeking behaviors will help inform the direction of future clinical research in the treatment of smoking. This research is funded by a R21 from NIDA (2022-2024).
Mechanisms of Opioid and Cocaine Polysubstance Addiction
Opioid use disorder (OUD) is a leading public health crisis in the United States that has led to a decrease in life expectancy. Individuals with OUD often use other substances, including cocaine. Stimulant use during opioid withdrawal may represent an attempt to ameliorate opioid withdrawal effects, but combined use of cocaine with opioids increases overdose risk. Results from previous studies in our lab illustrate glutamate as a conserved neural mechanism underlying drug-motivation in both opioid and cocaine use, as well as in polysubstance use. Our current research aims to develop targets for ameliorating glutamate dysregulation observed in comorbid addiction of these substances. This research is funded by a R21-R33 from NIDA (2020-2025).
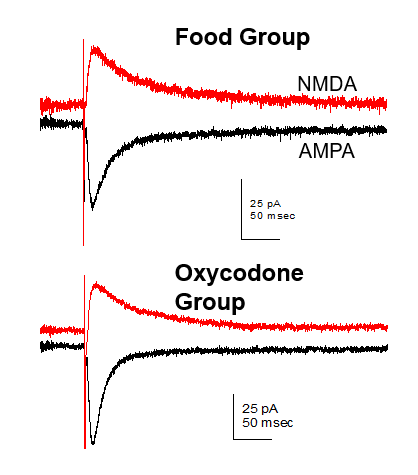
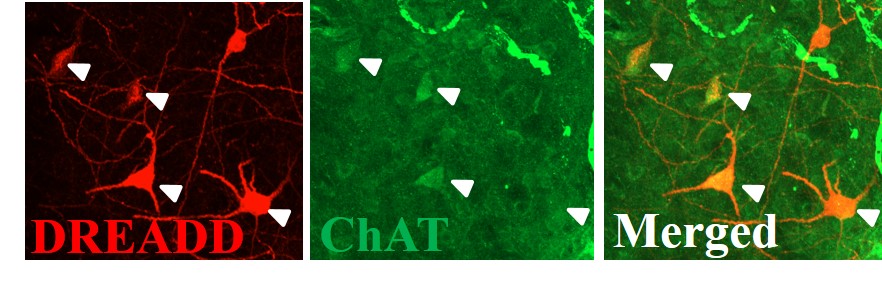
Mapping the Neural Circuity of Nicotine Addiction
This project examines the role of cholinergic interneurons within the nucleus accumbens in nicotine seeking motivation. Using specific chemogenic modulation of accumbens neuronal signaling in transgenic rats, we examine the link between signaling of different cell types to better our understanding of neural circuity underlying addiction-related behaviors. Further, we are interested in glutamatergic mechanisms, particularly how the disruption of glutamate homeostasis occurs in the drug-experienced brain. The resulting alteration of AMPA and NMDA receptor expression, affecting synaptic plasticity and neural communication, is also researched in our lab.